Why is sleep so interesting regarding migrating plains zebras?
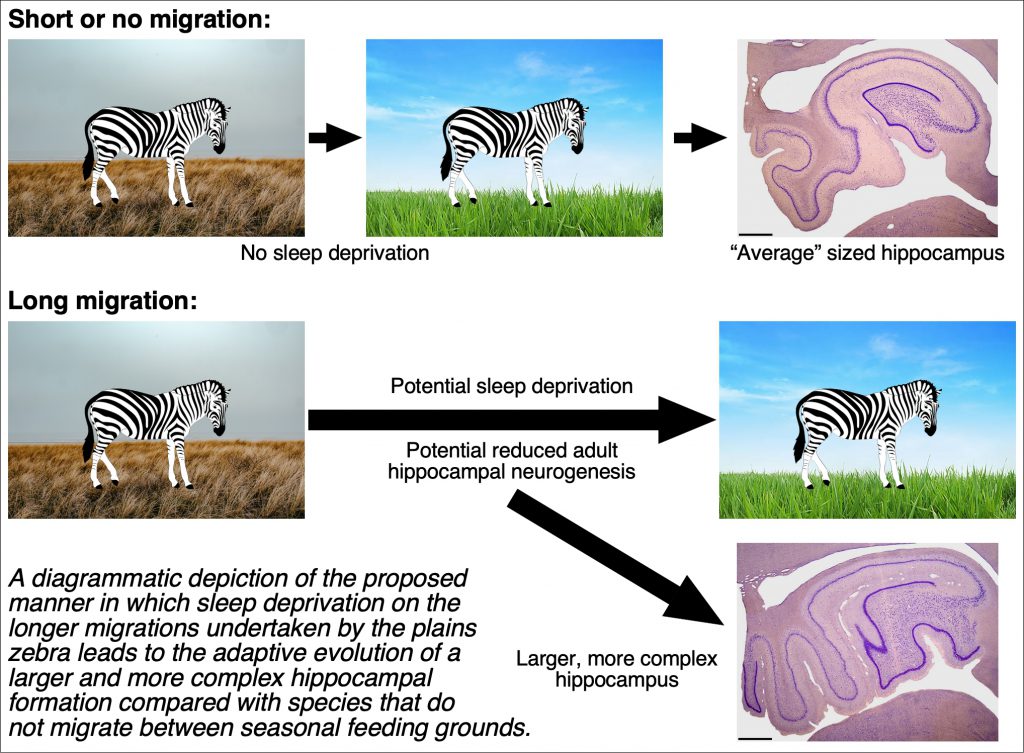
One aspect of mammalian biology that is often understated is the need to sleep – in undertaking this biological imperative the animals are vulnerable. During the migration the plains zebras traverse up to 24 km per day (Naidoo et al., 2016), significantly farther than their normal daily travel of less than 10 km (Klingel, 1967). The extra distance covered during the migration and the uncertainty of novel sleeping sites (the so-called ‘first night effect’, Agnew et al., 1966) will reduce the time available for sleep and rest. Thus, we propose that during the migration the zebras sacrifice the amount of time spent sleeping to allow them time to traverse a greater daily distance and time to obtain nutrition.
It is possible that during the evolutionary history of the plains zebra, there was a time point when movement from one seasonal feeding ground to another became necessary for survival. As the landscape slowly changed, and the distance between seasonal feeding grounds increased, the potential cognitive specialization of the hippocampus is likely to have evolved (around 370 000 years ago, Pedersen et al., 2018), allowing them to successfully navigate between increasingly distant seasonal feeding grounds. The proposed lack of sleep during migration will have several effects, but there are two effects of sleep deprivation we propose are related to the migration of plains zebras. First, sleep deprivation leads to significant declines in cognitive and memory recall capacities (Silva et al., 2004; Durmer and Dinges, 2005), and this will challenge navigational competency. This decreased navigational competency is likely compounded by the observation that sleep deprivation significantly lowers the rates of a process called adult hippocampal neurogenesis (AHN) (Meerlo et al., 2009), AHN likely being integral to navigational competency.
AHN, the process of adding new cells to the circuitry of the hippocampus in adult mammals, primarily occurs within the dentate gyrus of the hippocampus (Kempermann, 2012). The dentate gyrus is one of the main hubs through which the entorhinal cortex (home of the grid cells) is connected to the CA1 region of the hippocampus (home of the place cells). AHN has been observed in all mammals studied to date, apart from whales and dolphins (Patzke et al., 2015). We suggest that AHN is critical for the plains zebra as this process is likely to support the cognitive spatial map required for navigation by providing both functional plasticity and temporal updating of the environment in response to changes – environments in the real world are in a state of flux. While the full range of functions associated with AHN are not yet known, pattern separation is one of the key functions. Pattern separation is a neural process that allows the distinct representation of overlapping or similar inputs within the circuitry of the hippocampus (e.g., Sahay et al., 2011). Thus, the process of AHN can be proposed to allow the plains zebra to distinguish between similar features of the environment in its sedentary home ranges, and to update the spatial cognitive map in the sedentary home ranges and the migratory routes in response to environmental variations – but sleep deprivation will negatively affect this process.
The fact that the plains zebras successfully navigate their migratory routes, despite the potential sleep deprivation, may be offset by the evolutionary increase in size of the dorsal hippocampus – while not all neurons may be functioning at their full potential, enough neurons are likely to be functioning at levels sufficient to successfully maintain the navigational competency required for migration.
To test the idea that the plains zebra are sleep-deprived during the migration, and how this might help explain the evolution of the large hippocampus, we hope to examine sleep during the migration in a two-phased study.
Hypothesis: A lack of sleep during the migration, and thus a reduction in hippocampal function and AHN, forms the basis for the reactive, or compensatory, evolution of the large hippocampal formation in the plains zebra.
Aim: To provide an accurate description of sleep in the plains zebra, both when the animals are and are not migrating.
Specific Objective 1: Using subcutaneously implanted devices we aim to record polysomnographic and actigraphic parameters of sleep in plains zebras, to provide an overview of sleep and to validate actigraphy as an accurate method for the measurement of total sleep time in free-roaming and migrating plains zebra (e.g., Malungo et al., 2021).
Specific Objective 2: Using subcutaneously implanted devices, validated actigraphy techniques, and GPS collars (e.g., Gravett et al., 2017), we aim to determine whether the migration of the plains zebra alters its pattern of sleep by recording the validated actigraphic proxy of sleep during the southward migration of up to 20 plains zebras in Botswana.
To test this hypothesis, we propose to make a full polysomnographic study of sleep in plains zebras, during which actigraphy will be validated to physiological sleep. Following this we plan, using actigraphy, to study sleep in migrating zebras. If the hypothesis forwarded is supported, this being that sleep during the migration is compromised, we will be able to forward a conceptual argument regarding the evolution of the potential hippocampal navigational specialization observed in the plains zebra.
Methods:
Specific Objective 1: Using recently developed Neurologger technology (www.evolocus.com) we will record the electroencephalogram, nuchal electromyogram and electrooculogram in 6 (3 of each sex) adult plains zebra in a naturalistic setting (an enclosure of 20 hectares of their natural environment). Simultaneously, activity meters (Axy-4, TechnoSmArt, www.technosmart.eu) will be placed under the skin of the neck and hindleg to be able to correlate the recordings from these devices with the physiological parameters of sleep. This will validate the use of these devices for measuring sleep during the migration.
Specific Objective 2: To measure sleep during the migration, we plan to combine the use of 2 subcutaneously implanted Axy-4 activity meters (validated with physiological sleep as described above) in combination with GPS collar monitoring (to determine when the animals are migrating). We aim to implant and collar 20 plains zebra (10 of each sex) 4-6 weeks prior to the anticipated start of the southward migration in Botswana (Naidoo et al., 2016), determine when the migration occurs using GPS tracking, and then following the migration recover the implants to retrieve the recorded data. From the algorithms derived correlating actigraphic measures to physiological sleep (see above), the daily sleep quota for the animals prior to, during and after the migration will be determined.
Scientific sources of information:
Agnew HW, Webb WB, Williams RL (1966) The first night effect: an EEG study of sleep. Psychophysiol 2:263-266.
Durmer JS, Dinges DF (2005) Neurocognitive consequences of sleep deprivation. Seminars Neurol 25:117-129.
Gravett N, Bhagwandin A, Sutcliffe R, Landen K, Chase ML, Lyamin OI, Siegel JM, Manger PR (2017) Inactivity/sleep in two wild free-roaming African elephant matriarchs – does large body size make elephants the shortest mammalian sleepers? PLoS ONE 12: e0171903.
Kempermann G (2012) New neurons for ‘survival of the fittest’. Nat Rev Neurosci 13:727-736.
Klingel H (1967) Soziale Organisation und Verhalten freilebender Steppenzebras. Z Tierpsychol 24:580-624.
Malungo IB, Gravett N, Bhagwandin A, Davimes JG, Manger PR (2021) Sleep in two free-roaming blue wildebeest (Connochaetes taurinus), with observations on the agreement of polysomnographic and actigraphic techniques. IBRO Neurosci Rep 10:142-152
Meerlo P, Mistlberger RE, Jacobs BL, Heller HC, McGinty D (2009) New neurons in the adult brain: the role of sleep and consequences of sleep loss. Sleep Med Rev 13:187-194.
Naidoo R, Chase MJ, Beytell P, du Preez P, Landen K, Stuart-Hill G, Taylor R (2016) A newly discovered wildlife migration in Namibia and Botswana is the longest in Africa. Oryx 50:138-146.
Patzke N, Spocter M, Karlsson K, Bertelsen M, Haagensen M, Chawana R, Streicher S, Kaswera C, Gilissen E, Alagaili A, Mohammed O, Reep R, Bennett N, Siegel J, Ihunwo A, Manger P (2015) In contrast to many other mammals, cetaceans have relatively small hippocampi that appear to lack adult neurogenesis. Brain Struct Funct 220:361-383.
Pedersen CET, Albrechtsen A, Etter PD, Johnson EA, Orlando L, Chikhi L, Siegismund HR, Heller R (2018) A southern African origin and cryptic structure in the highly mobile plains zebra. Nature Ecol Evol 2:491-498.
Sahay A, Scobie KN, Hill AS, O’Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R (2011) Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature 472:466-470.
Silva RH, Abilio VC, Takatsu AL, Kameda SR, Grassi C, Chehin AB, Medrano WA, Calzavara MB, registro S, Andersen ML, Machado RB, Carvalho RC, Ribeiro RA, Tufik S, Frussa-Filho R (2004) Role of hippocampal oxidative stress in memory deficits induced by sleep deprivation in mice. Neuropharmacol 46:895-903.
